Medics4RareDiseases (M4RD) is a charity working to improve rare disease education among healthcare professionals by challenging them to change the way they think about the symptoms they see in their patients.
Rare diseases are collectively common – affecting 1 in 17 of us at some point in our lives – but with over 7,000 different rare diseases out there, it is impossible for anyone to be familiar with every single one. Instead of trying to train staff to identify every single disease, M4RD encourages healthcare professionals to ‘dare to think rare’
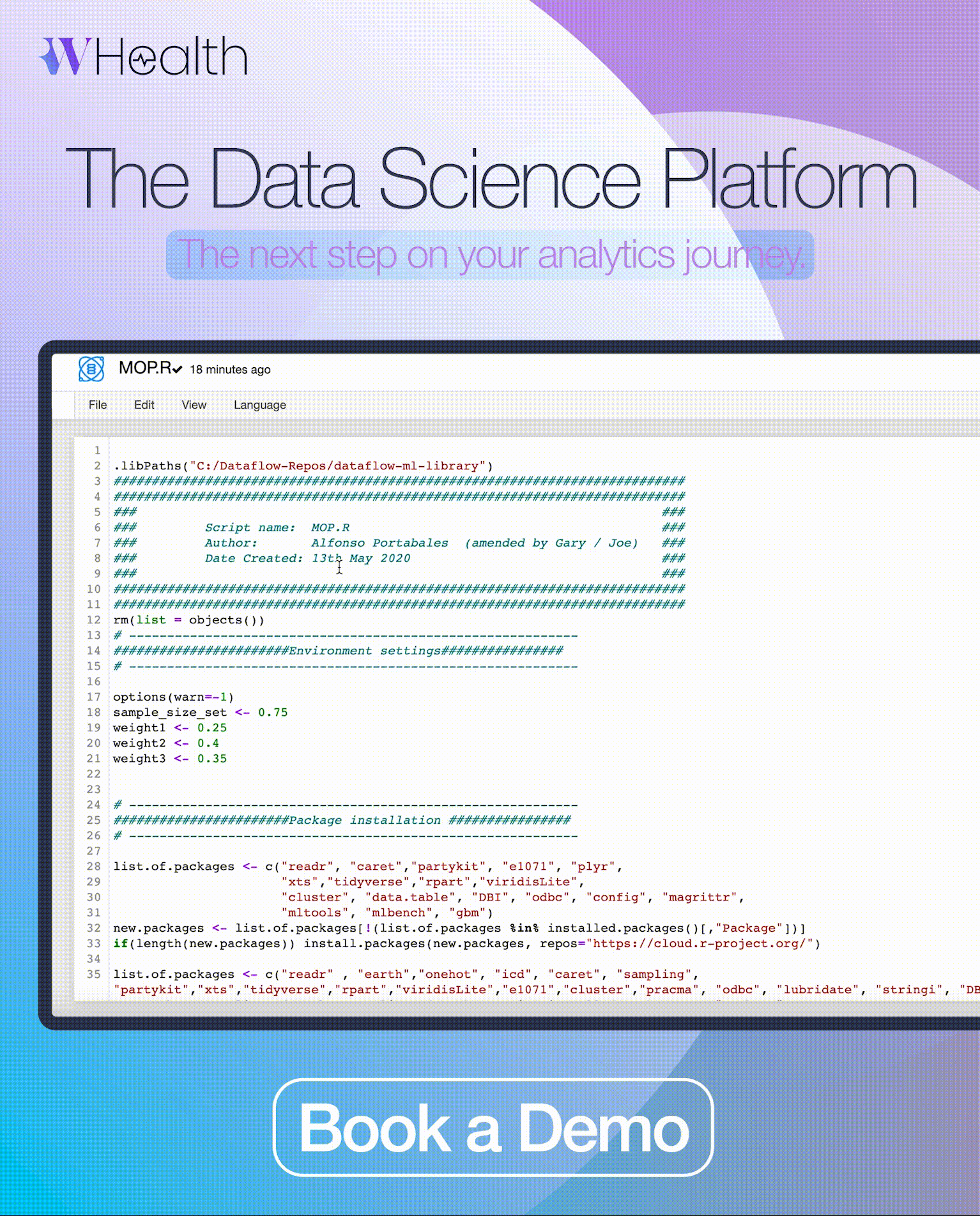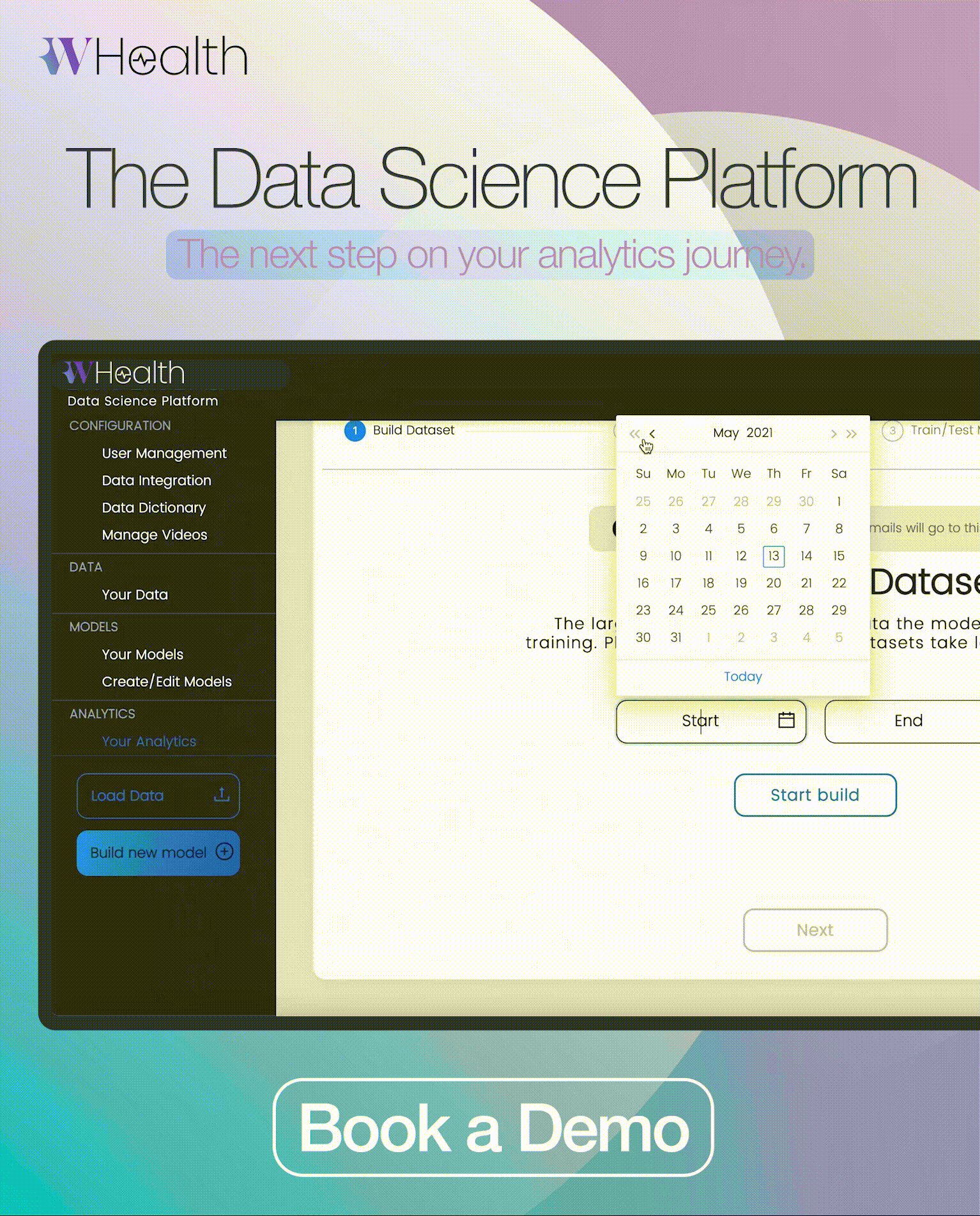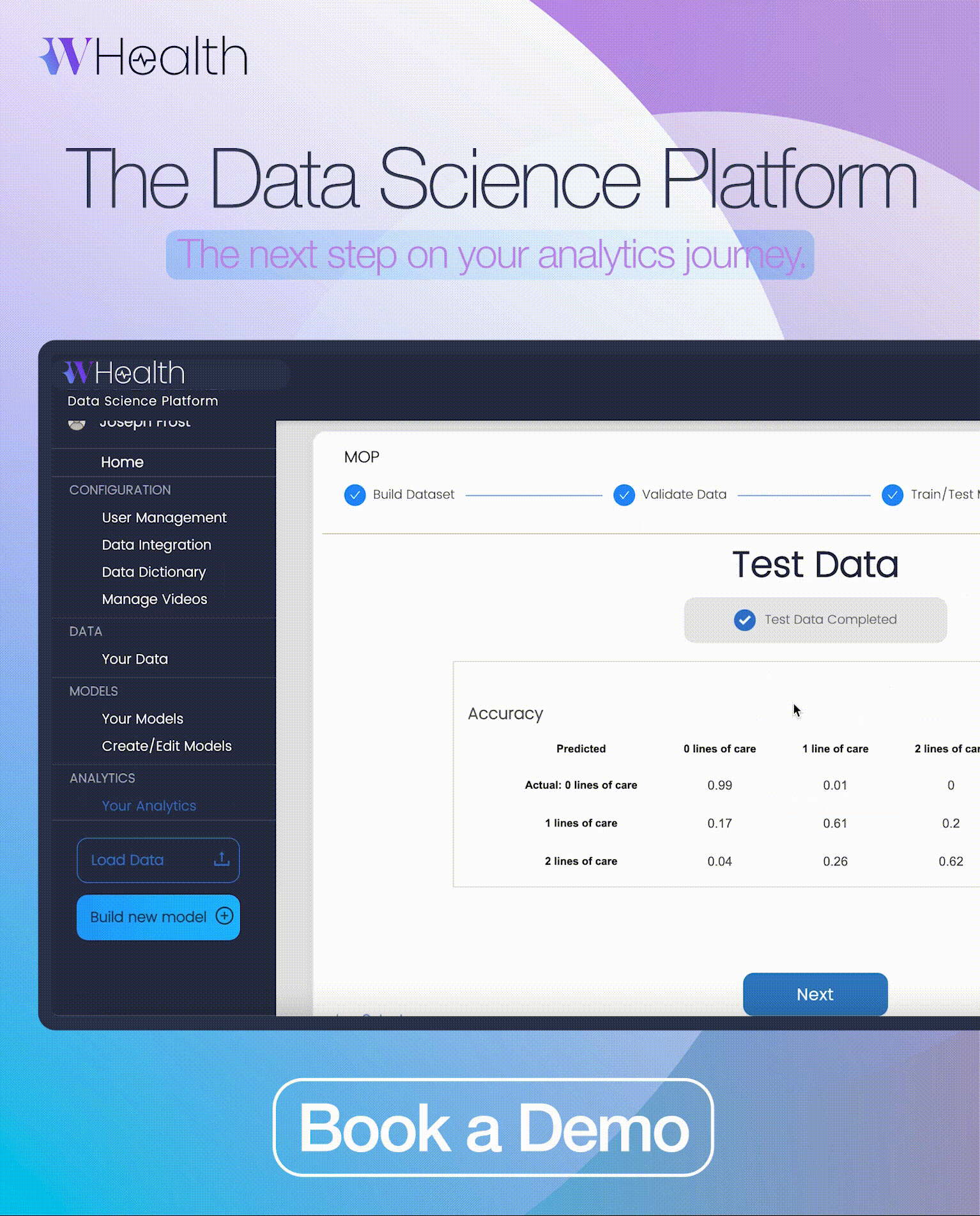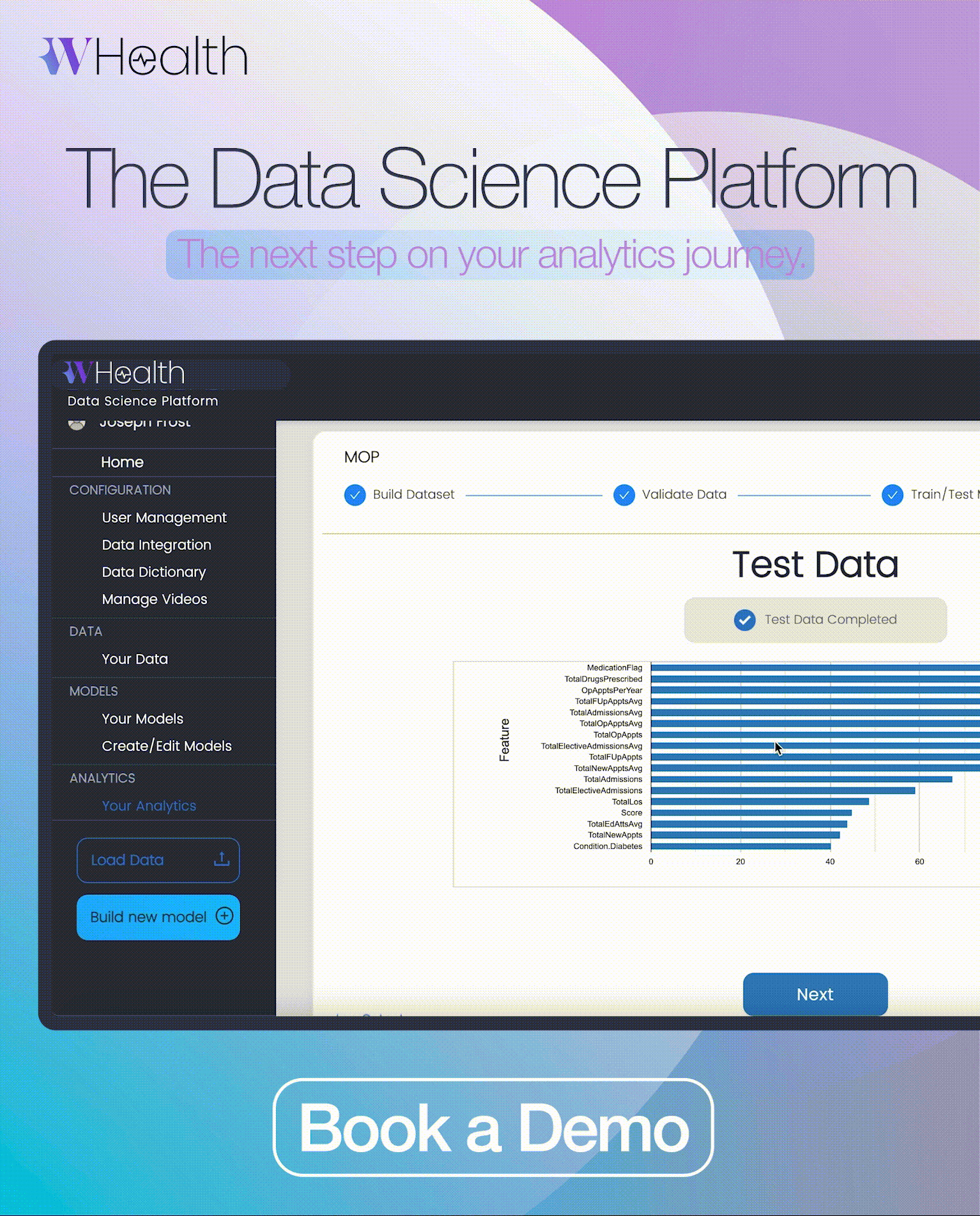
M4RD has now collaborated with global Contract Research Organisation Bionical Emas to launch new online training for healthcare professionals. This latest ‘mini-module is comprised of two lessons which provide essential education on clinical trials and early access programmes in the context of rare diseases.’
Early access programmes
Early access programmes such as the UK government’s Early Access Medicine Scheme (EAMS) allow patients to access promising innovative medicines before they receive wider market approval. Medications that are granted PIM (Promising Innovative Medicine) designation can be accessed via the scheme long before they are available to the wider public. Through EAMS, patient groups that have high unmet clinical needs, such as people with rare diseases, can access cutting edge therapies 12 to 18 months before they receive formal marketing authorisation.
For people with rare diseases, treatment options are often limited. People with Sickle Cell Disease, for example, have very few treatment options available to them despite the condition affecting around 15,000 people in the UK. A new treatment for SCD has recently been granted PIM designation and is now being made available to patients through EAMS. This is an incredible opportunity for patients to gain expedited access to potentially life-changing medications and for the real-world efficacy of the medication to be studied. However, many patients are understandably worried about taking medications that are yet to gain full approval. This is where M4RD’s latest mini-module can help.
M4RD’s clinical trial module
As a follow-up to the Rare Disease 101 module (which should be completed first), the new mini-module focuses on the ‘clinical trials and early access programmes are of immense importance to the rare disease community.’ The original Rare Disease 101 module aims to close the rare disease education gap and educate medical staff and students on the basics of rare diseases.
M4RD CEO Dr Lucky McKay commented that this new mini-module builds on ‘the basic principles that were covered in the original module: what rare disease is, when to suspect one, challenges faced by those living with these conditions and how to support them.’
Bionical Emas, the collaborator on this mini-module, provides a range of services to the pharmaceutical and biotech sector and M4RD will ‘draw on the organisation’s industry knowledge and expertise to diversify [their] online training offering and provide interesting and important new subjects.’
Improving healthcare providers’ familiarity with early access programmes and clinical trials in rare diseases is absolutely vital. Patients will look to the healthcare professionals around them for reassurance that these programmes are safe, so a clear understanding of how they work and how they can benefit patients is essential. Clinical trials are extensively monitored with intense vigilance throughout and patients are in very capable hands, but it is understandable that they may be worried when taking part. The knowledge gained from M4RD’s latest mini-module will leave providers able to signpost patients to relevant opportunities, clearly articulate the benefits, and assuage any worries they may have.
Recommended for you

Antidepressant Prescribing at Six-Year High
More people are taking antidepressants than ever. Is this a dark sign of the times or an indication that mental health stigma is changing?

Can AI be Used to Determine Cancer Recurrence?
When cancer patients go into remission, they often worry about it coming back. AI can now help identify those at risk of cancer recurrence.

Pegasus – Still a Threat to the UK?
The notorious Pegasus spyware has been misused to exploit vulnerabilities in devices, even those kept within the walls of Number 10.
Trending

Drug Decriminalisation: Could the UK Follow Portugal?
Portugal’s drug decriminalisation has reduced drug deaths and made people feel safe seeking support. Would the UK ever follow suit?

Calling All Unvaccinated UK Adults
With Covid cases rising, the NHS is urging the 3 million UK adults who remain unvaccinated to come forward.